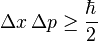By the end of the 19th century, Maxwell's theory of electromagnetism wasn't able to explain several phenomena, such as:
- Black body radiation;
- Photoelettric effect;
- Compton scattering;
- Emission and adsorption spectrum of atoms.
Those phenomena were interpreted using quantum mechanics, that was based on the idea that light could behave in a particle way, and not only as a wave. Max Plank was the first one to introduce the idea of quantization, that allowed to explain black body radiation.
If we take in account any metal that melts at high temperatures, we'll see that he emits radiation in a continuous spectrum, that varies with temperature. Black body is a theoretical object that behaves in a similar way: it adsorbe all the incoming radiation, and its emission depends only on temperature. Its spectrum will present a maximal emission at a certain wavelength, that will vary according to Wien's Law.
In 1899 Max Plank succeeded in interpretating the experimental results about black body radiation. He assumed that energy could not vary in a continuous way, but that it was quantized.
In 1905 use this new idea to solve the paradox of Photoelectric effect. This effect was based on the fact that electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. On experiments it was observed that electron emission took place only if the incident radiation had a bigger frequency than a certain minimum one (typical of the irradiated body). The emitted electrons had a certain kinetic energy, that varied from zero, to a maximum value, dependent from the frequency of the radiation. The intensity of emitted electrons was proportional to incident radiation's intensity, meanwhile their speed (and so their kinetic energy) was independent from it.
According to classical physics, electrons of superficial layers could be stimulated by incident radiation, but the speed of emitted electrons should vary proportionally with the intensity of incident radiation. This was in contrast with experimental results, so a new conception of electromagnetic waves was needed.
Einstein proposed that electromagnetic waves, while interacting with matter, had a corpuscular behavior, as if it was composed by quanta of light, photons. While intensity of radiation increases, photon's energy remains the same, and gains the number of photons per unity of surface. A photon can indeed give his energy to surface electron: if it is bigger than the minimum required, the electron will be emitted, and will assume a kinetic energy equal to the difference of photon's kinetic energy and the minimum energy required.
This explains why to an increasing intensity of incident radiation, corresponds a gaining of emitted electrons, while their kinetic energy is not changed. This interpretation led to the introduction of wave particle duality of light.