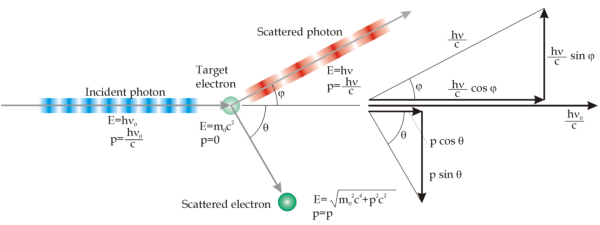
Compton proposed that this phenomena could be explained as an interaction between particles: photons and electrons. Photons, interacting with graphite, are going to hit electrons, shifting part of they energy. The electron is therefore going to be emitted with a lower kinetic energy than the photon. It means that also the frequency will be lower, thus the wavelength will be higher, explaining the Compton scattering.
This new discovery, together with photoelectric effect, showed that light behaved ad a particle. De Broglie, was firmly convinced about unity in nature: if waves behaved like matter, also matter had to behave like waves. In 1924 he proposed that the motion of particles was related to the propagation of a certain wave, according to his hypothesis:
This was experimentally proved in 1927 by Davidson and Germer. For instance if an electromagnetic wave is forced to pass through a hole with radius comparable to it's wavelenght, it will create diffraction. The same behavior is observed if electrons are forced to pass through a hole of radius comparable to their de broglie-wavelength, confirming wave particle duality of light and matter.





3 comments:
What you're saying is completely true. I know that everybody must say the same thing, but I just think that you put it in a way that everyone can understand. I'm sure you'll reach so many people with what you've got to say.
I really appreciate your professional approach. These are pieces of very useful information that will be of great use for me in future.
I certainly agree to some points that you have discussed on this post. I appreciate that you have shared some reliable tips on this review.
Post a Comment