The problem that was being posed now was if it could be possible to elaborate models that required the existence of precise orbits. To calculate the path of a certain dot, it necessary to know its position and speed at a certain moment.
In response to this problem Heisenberg proposed the uncertainty principle, according to which is not possible to measure accurately and contemporaneously the position and the speed of a certain particle
It means that lower is the radiation's intensity used to observed the particle, grater is the accuracy of the measurement, because the particle's path is going to be effected in a minor way. However to observe the path of an electron, a radiation of wavelength comparable to its dimension is required. This implies a very small wavelength, that is related to high energy, and therefore great change in particle's speed, that is several cases, will be sufficient to ionize the atom.
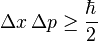




3 comments:
Hey keep posting such good and meaningful articles.
Very interesting tips shared by you. I really like to read your blogs as they contains very important and informative content. I appreciate your efforts. Cheers.
Very informative, keep posting such good articles, it really helps to know about things.
Post a Comment